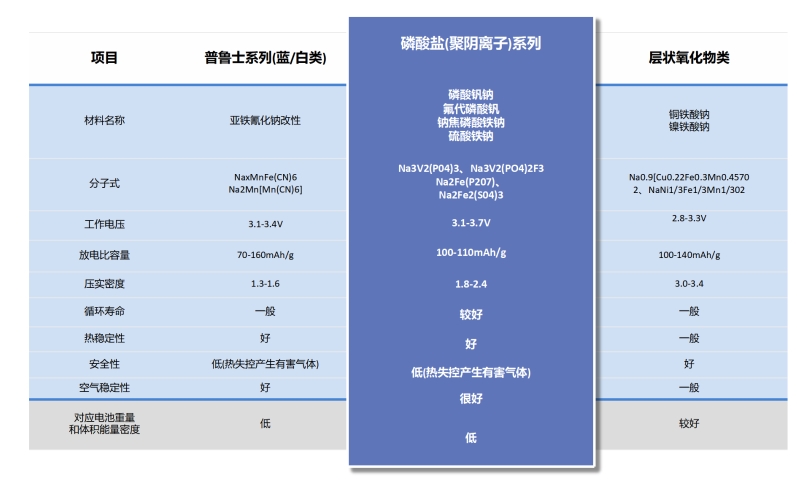
Polyanionic compounds are also key materials for positive electrode materials in sodium ion batteries, and their rich variety and unique sodium storage performance have attracted the attention of researchers. Polyanionic materials refer to compounds with a series of tetrahedral (XO4) n structures? Anionic unit and its derivative unit (XmO3m+1) n? A type of compound composed of (X=S, P, Si, As, Mo or W) and polyhedral unit MOx (M represents transition metal). In most polyanionic compounds, (XO4) n? Anionic units not only allow ions to conduct quickly within an open structural framework, but also stabilize the redox pairs of transition metals. Compared with layered compounds, the strong covalent bond of X-O in polyanionic compounds can induce stronger ionization of M-O covalent bonds, resulting in higher transition metal redox pairs. This is the "induction effect" in polyanionic compounds, therefore polyanionic electrode materials often have higher operating voltages. Moreover, the strong covalent bond between X and O stabilizes O in the lattice, making polyanionic materials often have high structural stability and safety. This is also one of the reasons why polyanionic materials are more suitable for rechargeable secondary batteries.
Compared with oxides or other types of positive electrode materials, the significant advantages of polyanionic compounds are their stable three-dimensional structure, wide voltage platform, and high safety. For example, phosphate materials have P-O covalent bonds, which give them high thermal stability. It is very common for layered oxides to decompose and release oxygen at temperatures above 200 ℃, and the covalent bonds of polyanionic compounds can effectively suppress this problem. However, compared with layered oxides and Prussian blue, the conductivity of polyanionic compounds is generally poor. Carbon coating is more frequently used in surface modification of polyanionic compounds than other materials, and to some extent, it improves the conductivity of such materials. Another major problem with polyanionic compounds is their strong water absorption ability. When the surface comes into contact with water, it generates NaOH, and an uneven material surface may have a negative impact on the electrochemical performance of the electrode.
Taking phosphate based NaFePO4 as an example, currently there are four main modification methods for improving the conductivity and energy density of polyanionic compound positive electrode materials in sodium ion batteries: (1) controlling the sodium storage capacity in the sodium storage sites of polyanionic compounds; (2) Using transition metal elements to partially or completely replace Fe elements; (3) Prepare a mixed polyanion system with F, OH, and CO2; (4) Combined phosphate (PO4) 3? And pyrophosphate (P2O7) 4? To stabilize the crystal structure. However, they generally have the disadvantage of low electronic conductivity and require carbon coating to improve their electrochemical performance. Common polyanionic cathode materials mainly include phosphates, pyrophosphates, sulfates, silicates, borate salts, and mixed polyanions.
1. The phosphate type polyanionic compound NaMPO4 with olivine structure has the simplest structure among polyanionic materials. This material has electrochemical activity based on the redox properties of Fe2+/3+. However, the low capacity and stability of this material itself make it somewhat inferior to practical cathode materials.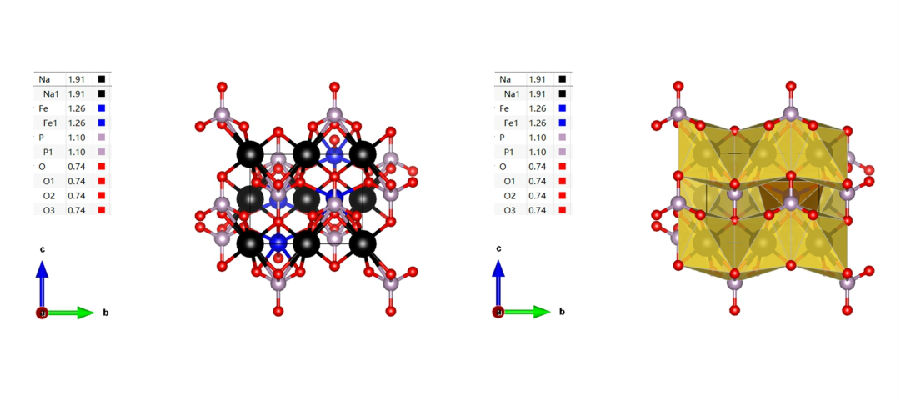
For NaFePO4, the olivine phase can only exist stably below 480 ℃, while its thermodynamically stable phase belongs to the phosphate iron sodium ore phase when the temperature exceeds 480 ℃. As shown in the above figure, the NaFePO4 with olivine structure belongs to the orthorhombic crystal system, with a spatial group of Pmnb. The crystal is composed of FeO6 octahedra and PO4 tetrahedra, forming a spatial framework. Na+occupies a common octahedral position and forms long chains along the b-axis direction. One FeO6 octahedron shares an edge with two NaO6 octahedrons and one PO4 tetrahedron, while the PO4 tetrahedron shares an edge with one FeO6 octahedron and two NaO6 octahedrons. Sodium ions have one-dimensional transport channels, and during the charging and discharging process, sodium ions can easily detach/embed without damaging the main structure. NaFePO4 has also been widely studied and applied as a positive electrode sodium storage material in sodium ion batteries. Setting the charging and discharging range in organic systems to the same voltage range as stable charging and discharging in water results in better polarization and rate performance of NaFePO4 in aqueous batteries. This may be related to the superior ion conductivity and interface impedance of water compared to organic systems. Therefore, it can be considered that olivine type NaFePO4 is more suitable as the positive electrode material for aqueous sodium ion batteries. 2. Pyrophosphate type polyanionic compounds obtained by replacing phosphate ions with pyrophosphate ions are widely used in lithium-ion batteries, and the same research approach can also be applied to sodium ion batteries. Sodium based pyrophosphate materials are mainly divided into two categories: NaMP2O7 (M=Ti, V, Fe) and Na2MP2O7 (M=Fe, Mn, Co), and each material may have multiple crystal types at the same time. For example, NaMP2O7 contains various structures such as triclinic, monoclinic, or tetragonal, all of which can provide channels for Na+migration. When used as the positive electrode for sodium ion batteries, excess raw materials can prepare sodium rich structures. Therefore, even without carbon coating and nanoscale structures, this material can exhibit good electrochemical performance compared to other phosphate materials. 3. Sulfate type polyanionic compounds have stronger electronegativity than phosphate ions. Therefore, using sulfate ions to replace phosphate ions can improve the working voltage of the material as the positive electrode of sodium ion batteries. In NaMSO4F materials, M can also be transition metal compounds such as Ni and Co, which share the common feature of improving the low operating voltage and low conductivity of fluorophosphates to a certain extent. In future research, various transition metal based polyanionic compounds incorporating sulfate ions will play an important role.
There are various types and forms of polyanionic compounds. Compared with layered oxides, polyanionic compounds can not only introduce various transition metal elements, but also combine multiple anionic groups together, providing researchers with more research ideas through this diverse combination method. The anionic electronegativity in polyanionic compounds is relatively high, so this type of material generally has a higher working voltage when used as a positive electrode material for sodium ion batteries. Overall, polyanionic sodium ion battery cathode materials have shown enormous potential for application in the field of new energy due to their unique advantages.